
Top Performing Drug of 2021 - Eliquis (July Edition)
Active Ingredients: Apixaban
Strength: 2.5 mg and 5 mg
Dosage Form: Tablet
Mechanism of Action: Factor Xa inhibitors
First Approval: US (Dec 28, 2012), EU (May 18, 2011)
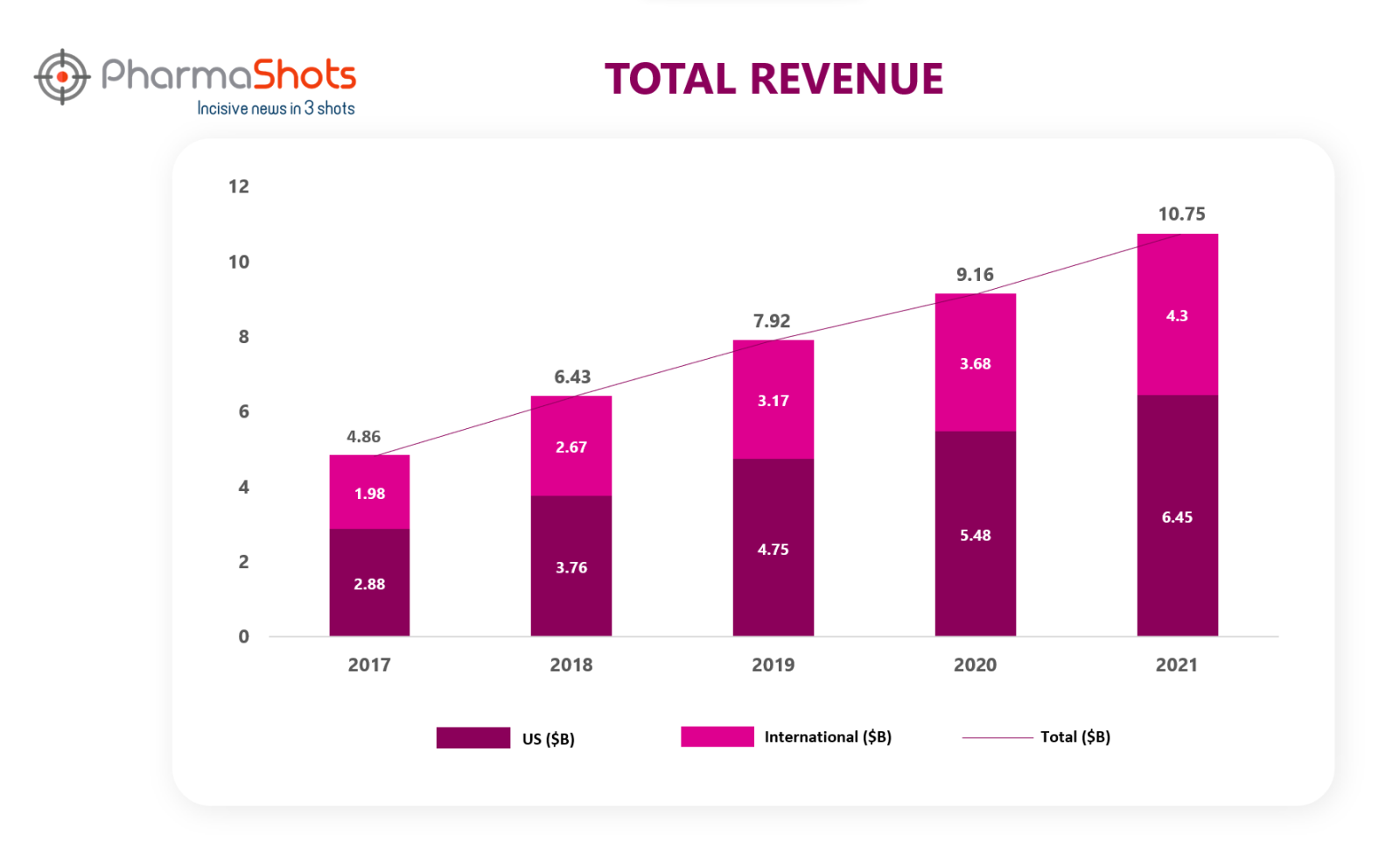
Revenue1
Eliquis is a leading novel oral anticoagulant of BMS. The revenue for BMS continued to grow due to Eliquis and other recently launched new products. BMS and Pfizer jointly develop and commercialize Eliquis. Pfizer funds between 50% and 60% of all development costs depending on the study sharing profits and losses are shared equally on a global basis except in certain countries. Worldwide sales of Eliquis increased by 17% in 2021, 16% in 2020, 23% in 2019, 32% in 2018 and 46% in 2017. Let’s explore in what manner the revenue of Eliquis changed over the last five years.1
Approved Indications for Eliquis2
Eliquis is approved and marketed in the US and Europe for various indications including
- Stroke And Systemic Embolism in Nonvalvular Atrial Fibrillation: To reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
- Deep Vein Thrombosis Following Hip or Knee Replacement Surgery: For the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in patients who have undergone hip or knee replacement surgery
- Treatment Of Deep Vein Thrombosis: For the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and Pulmonary Embolism following initial therapy
Clinical Trials3
Eliquis has a total of 142 trials, including 69 industry trials of which 47 were interventional, 21 observational & 1 expanded access trial. From the analysis of clinical trials, we have made a representation for the trials of Eliquis. (Trials are taken as of 19 July 2022).
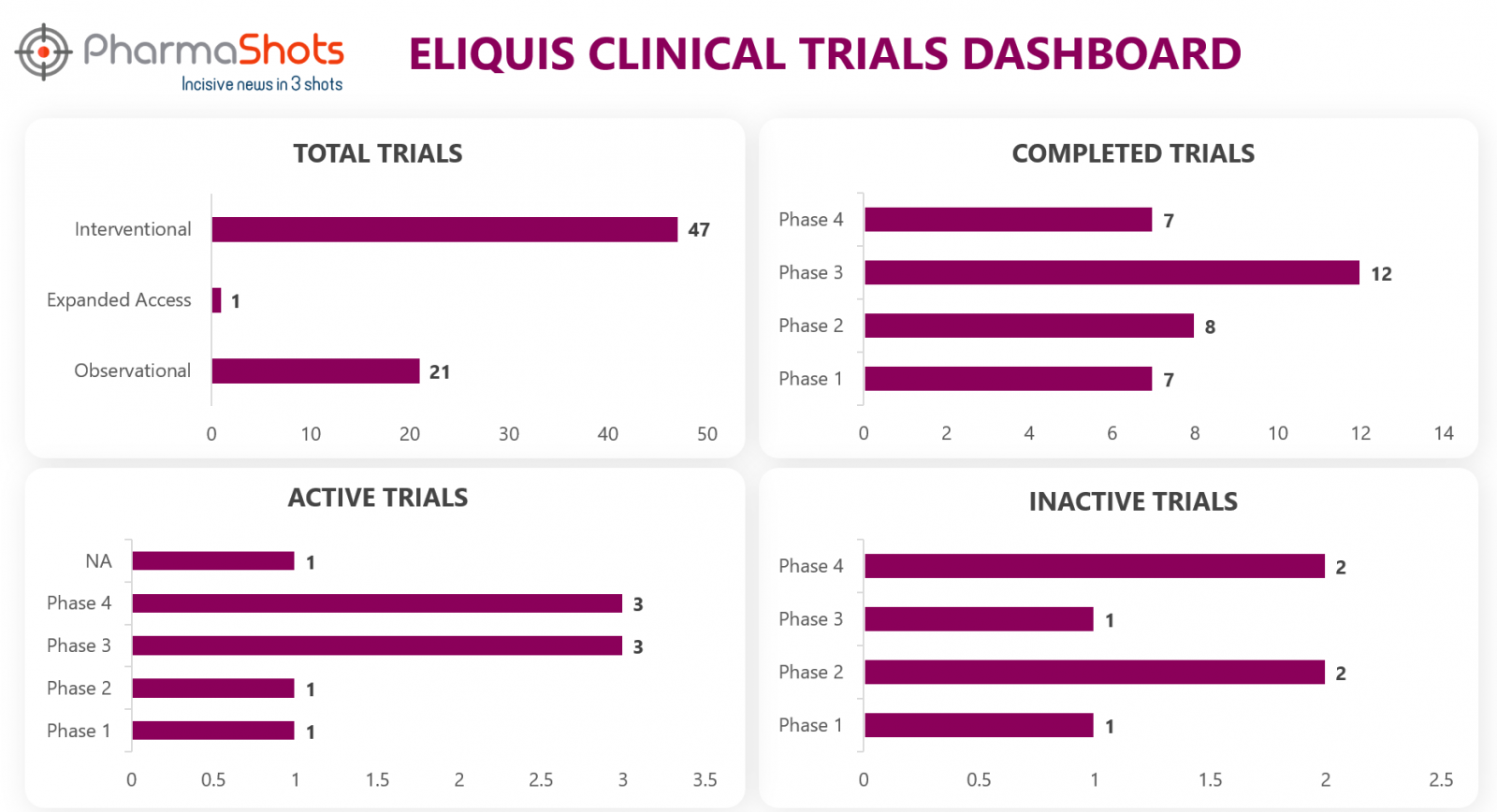
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status

Get help paying for your medicine4
- BMS Patient Support Program helps patients to understand their coverage of the insurance plan covering BMS medicines. This includes benefits investigation and prior authorization support, as well as out-of-pocket costs
- Some Bristol Myers Squibb medications are available free of charge. This helps patients who could meet their needs financially or do not have insurance
ELIQUIS 360 SUPPORT5
ELIQUIS 360 Support helps patients to understand prescription coverage of Eliquis. This program also provides:
- Helpful information to know about your condition
- Request to activate co-pay card
- Talking to live specialists to know about your health conditions and taking Eliquis.
Eliquis Co-Pay Card
- After activation of the card, eligible participants with a valid prescription for ELIQUIS at participating pharmacies may pay as little as $10 per 30-day supply (up to 74 tablets for the first fill, and up to 60 tablets for all subsequent fills) for up to 24 months, subject to a maximum annual benefit of $6,400.
Eliquis free trial offer card
- After the activation of the card, eligible patients with a Free 30-Day Trial card and a 30-day prescription for ELIQUIS at participating pharmacies can receive a free 30-day supply (up to 74 tablets) of ELIQUIS.
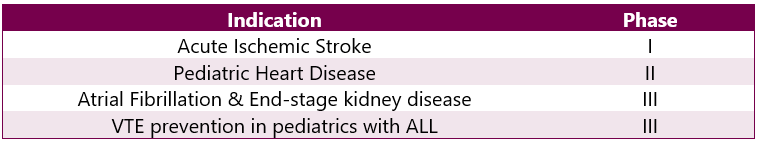
Eliquis Pipeline Analysis6
Various trials are ongoing for other indications. This can be categorized as follows:
Patents7
Patents have long been considered essential determinants advancing innovation, particularly the development of new prescription drugs. Eliquis revenue has been growing constantly due to its higher demand. Losing the Eliquis patent fight would have been enormously overpriced for Bristol Myers Squibb and Pfizer. The company has settled various patent litigations and generic challengers for Eliquis in 2021.
Eliquis has a total of 168 international patents in 42 countries.
Eliquis Generics8
The FDA approved the first-ever generic versions of Eliquis in Dec 2019 with dosages available at 2.5mg and 5mg for patients who are at high risk for stroke, pulmonary embolism (PE), and deep vein thrombosis (DVT).
References:
- BMS 10-K
- Eliquis - Prescribing Information
- Clinicaltrials.gov
- Eliquis Patient support
- Eliquis - savings and support
- BMS - Pipeline
- Drugpatentwatch - Eliquis
- Eliquis - FDA generics approval
Related Post: Top Performing Drug of 2021 - Revlimid (June Edition)
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.














